 English
English



-
 Afrikaans
Afrikaans -
 Albanian
Albanian -
 Amharic
Amharic -
 Arabic
Arabic -
 Armenian
Armenian -
 Azerbaijani
Azerbaijani -
 Basque
Basque -
 Belarusian
Belarusian -
 Bengali
Bengali -
 Bosnian
Bosnian -
 Bulgarian
Bulgarian -
 Catalan
Catalan -
 Cebuano
Cebuano -
 China
China -
 China (Taiwan)
China (Taiwan) -
 Corsican
Corsican -
 Croatian
Croatian -
 Czech
Czech -
 Danish
Danish -
 Dutch
Dutch -
 English
English -
 Esperanto
Esperanto -
 Estonian
Estonian -
 Finnish
Finnish -
 French
French -
 Frisian
Frisian -
 Galician
Galician -
 Georgian
Georgian -
 German
German -
 Greek
Greek -
 Gujarati
Gujarati -
 Haitian Creole
Haitian Creole -
 hausa
hausa -
 hawaiian
hawaiian -
 Hebrew
Hebrew -
 Hindi
Hindi -
 Miao
Miao -
 Hungarian
Hungarian -
 Icelandic
Icelandic -
 igbo
igbo -
 Indonesian
Indonesian -
 irish
irish -
 Italian
Italian -
 Japanese
Japanese -
 Javanese
Javanese -
 Kannada
Kannada -
 kazakh
kazakh -
 Khmer
Khmer -
 Rwandese
Rwandese -
 Korean
Korean -
 Kurdish
Kurdish -
 Kyrgyz
Kyrgyz -
 Lao
Lao -
 Latin
Latin -
 Latvian
Latvian -
 Lithuanian
Lithuanian -
 Luxembourgish
Luxembourgish -
 Macedonian
Macedonian -
 Malgashi
Malgashi -
 Malay
Malay -
 Malayalam
Malayalam -
 Maltese
Maltese -
 Maori
Maori -
 Marathi
Marathi -
 Mongolian
Mongolian -
 Myanmar
Myanmar -
 Nepali
Nepali -
 Norwegian
Norwegian -
 Norwegian
Norwegian -
 Occitan
Occitan -
 Pashto
Pashto -
 Persian
Persian -
 Polish
Polish -
 Portuguese
Portuguese -
 Punjabi
Punjabi -
 Romanian
Romanian -
 Russian
Russian -
 Samoan
Samoan -
 Scottish Gaelic
Scottish Gaelic -
 Serbian
Serbian -
 Sesotho
Sesotho -
 Shona
Shona -
 Sindhi
Sindhi -
 Sinhala
Sinhala -
 Slovak
Slovak -
 Slovenian
Slovenian -
 Somali
Somali -
 Spanish
Spanish -
 Sundanese
Sundanese -
 Swahili
Swahili -
 Swedish
Swedish -
 Tagalog
Tagalog -
 Tajik
Tajik -
 Tamil
Tamil -
 Tatar
Tatar -
 Telugu
Telugu -
 Thai
Thai -
 Turkish
Turkish -
 Turkmen
Turkmen -
 Ukrainian
Ukrainian -
 Urdu
Urdu -
 Uighur
Uighur -
 Uzbek
Uzbek -
 Vietnamese
Vietnamese -
 Welsh
Welsh -
 Bantu
Bantu -
 Yiddish
Yiddish -
 Yoruba
Yoruba -
 Zulu
Zulu
Electrochemical Analysis of Precipitation Titration Techniques for Ion Detection and Measurement
Potentiometric Precipitation Titration An Overview
Potentiometric precipitation titration is a sophisticated analytical technique utilized for the determination of the concentration of specific ions in a solution. This method combines the principles of potentiometry—a technique that measures the voltage of electrochemical cells— with precipitation reactions, wherein a solute forms an insoluble compound when it reacts with another substance in solution. This article delves into the details of this method, its principles, applications, and advantages.
Principles of Potentiometric Precipitation Titration
At the core of potentiometric precipitation titration is the measurement of the potential change that occurs as a titrant is added to a sample solution. The titrant is typically a reagent that reacts with the analyte to form a precipitate. As the reaction proceeds, the concentration of free ions in the solution decreases, which influences the electrical potential measured by an ion-selective electrode.
A key component of this method is the use of a suitable reference electrode and the titrant, which often includes compounds like silver nitrate (AgNO₃) or barium chloride (BaCl₂). For example, in the titration of chloride ions (Cl⁻), silver nitrate is added, leading to the formation of insoluble silver chloride (AgCl) as a precipitate. The electrochemical cell's potential will reflect the changes in the concentration of chloride ions until the equivalence point is reached—when all the chloride ions have reacted with the silver ions to form a complete precipitate of AgCl.
Procedure
The procedure for potentiometric precipitation titration typically involves the following steps
1. Preparation of Sample The solution containing the analyte is prepared, possibly requiring dilution or adjustment of pH to optimize the precipitation reaction. 2. Electrode Setup A suitable reference electrode and ion-selective electrode are placed in the solution. The electrodes determine the potential of the solution throughout the titration process.
3. Titration The titrant is gradually added to the sample while continuously monitoring the potential change. The data collected is plotted on a graph of potential versus volume of titrant added.
4. Determination of Equivalence Point The equivalence point can be determined by observing inflection points in the titration curve, where a sharp change in potential occurs. This point indicates the completion of the precipitation reaction.
5. Calculations Finally, the concentration of the analyte can be calculated using the titration data and appropriate stoichiometric relationships.
Applications
potentiometric precipitation titration
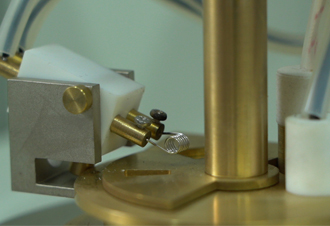
Potentiometric precipitation titration finds a variety of applications across different fields
- Environmental Analysis This method is useful in assessing water quality by determining the concentration of halides or heavy metals that can form precipitates
.- Pharmaceuticals In the pharmaceutical industry, it is used to analyze the purity of drug compounds by measuring the concentration of specific ions.
- Food Industry This technique can also monitor food quality, particularly in determining the levels of salts in food products.
Advantages
The potentiometric precipitation titration method offers several advantages
1. Sensitivity This method can detect low concentrations of ions, making it suitable for trace analysis.
2. Precision The potential measurement allows for accurate determination of concentration, which is crucial in both research and industrial applications.
3. Automation Potential With advancements in technology, potentiometric titration methods can be automated, leading to increased efficiency and reproducibility.
4. Minimal Sample Preparation Compared to other analytical techniques, the sample preparation required is often less complex, saving time and resources.
Conclusion
In summary, potentiometric precipitation titration is a powerful analytical technique that provides accurate and sensitive measurements of ion concentrations in various samples. Its strategic combination of potentiometry and precipitation reactions enhances its applicability across numerous fields, from environmental science to pharmaceuticals. As technology continues to evolve, the integration of automation and refined analytical methods will likely enhance the efficiency and scope of this valuable technique even further.
-
Testing Equipment Industry Sees Major Advancements in 2025: Smart & Precision Technologies Lead the WayNewsJun.06,2025
-
Applications of Direct Current Generators in Renewable Energy SystemsNewsJun.05,2025
-
Hipot Tester Calibration and Accuracy GuidelinesNewsJun.05,2025
-
Digital Circuit Breaker Analyzer Features and BenefitsNewsJun.05,2025
-
Benefits of Real-Time Power Quality Monitoring Devices for Industrial EfficiencyNewsJun.05,2025
-
Earth Fault Loop Testing in High-Rise Building Electrical SystemsNewsJun.05,2025



