 English
English



-
 Afrikaans
Afrikaans -
 Albanian
Albanian -
 Amharic
Amharic -
 Arabic
Arabic -
 Armenian
Armenian -
 Azerbaijani
Azerbaijani -
 Basque
Basque -
 Belarusian
Belarusian -
 Bengali
Bengali -
 Bosnian
Bosnian -
 Bulgarian
Bulgarian -
 Catalan
Catalan -
 Cebuano
Cebuano -
 China
China -
 China (Taiwan)
China (Taiwan) -
 Corsican
Corsican -
 Croatian
Croatian -
 Czech
Czech -
 Danish
Danish -
 Dutch
Dutch -
 English
English -
 Esperanto
Esperanto -
 Estonian
Estonian -
 Finnish
Finnish -
 French
French -
 Frisian
Frisian -
 Galician
Galician -
 Georgian
Georgian -
 German
German -
 Greek
Greek -
 Gujarati
Gujarati -
 Haitian Creole
Haitian Creole -
 hausa
hausa -
 hawaiian
hawaiian -
 Hebrew
Hebrew -
 Hindi
Hindi -
 Miao
Miao -
 Hungarian
Hungarian -
 Icelandic
Icelandic -
 igbo
igbo -
 Indonesian
Indonesian -
 irish
irish -
 Italian
Italian -
 Japanese
Japanese -
 Javanese
Javanese -
 Kannada
Kannada -
 kazakh
kazakh -
 Khmer
Khmer -
 Rwandese
Rwandese -
 Korean
Korean -
 Kurdish
Kurdish -
 Kyrgyz
Kyrgyz -
 Lao
Lao -
 Latin
Latin -
 Latvian
Latvian -
 Lithuanian
Lithuanian -
 Luxembourgish
Luxembourgish -
 Macedonian
Macedonian -
 Malgashi
Malgashi -
 Malay
Malay -
 Malayalam
Malayalam -
 Maltese
Maltese -
 Maori
Maori -
 Marathi
Marathi -
 Mongolian
Mongolian -
 Myanmar
Myanmar -
 Nepali
Nepali -
 Norwegian
Norwegian -
 Norwegian
Norwegian -
 Occitan
Occitan -
 Pashto
Pashto -
 Persian
Persian -
 Polish
Polish -
 Portuguese
Portuguese -
 Punjabi
Punjabi -
 Romanian
Romanian -
 Russian
Russian -
 Samoan
Samoan -
 Scottish Gaelic
Scottish Gaelic -
 Serbian
Serbian -
 Sesotho
Sesotho -
 Shona
Shona -
 Sindhi
Sindhi -
 Sinhala
Sinhala -
 Slovak
Slovak -
 Slovenian
Slovenian -
 Somali
Somali -
 Spanish
Spanish -
 Sundanese
Sundanese -
 Swahili
Swahili -
 Swedish
Swedish -
 Tagalog
Tagalog -
 Tajik
Tajik -
 Tamil
Tamil -
 Tatar
Tatar -
 Telugu
Telugu -
 Thai
Thai -
 Turkish
Turkish -
 Turkmen
Turkmen -
 Ukrainian
Ukrainian -
 Urdu
Urdu -
 Uighur
Uighur -
 Uzbek
Uzbek -
 Vietnamese
Vietnamese -
 Welsh
Welsh -
 Bantu
Bantu -
 Yiddish
Yiddish -
 Yoruba
Yoruba -
 Zulu
Zulu
Understanding the Importance of Titration in Chemical Analysis and Laboratory Practices
Understanding Titration Tests An Essential Skill in Chemistry
Titration is a fundamental laboratory technique primarily used in chemistry to determine the concentration of an unknown solution through a controlled reaction between it and a titrant of known concentration. This process not only provides valuable quantitative analysis but also sharpens essential skills in laboratory practice. The significance of titration tests extends into various fields, including pharmaceuticals, environmental testing, and food safety.
At its core, titration involves delivering a titrant—usually a solution with a precisely known concentration—into a flask containing the analyte (the unknown solution) until the reaction reaches its endpoint. The endpoint is typically indicated by a color change, which can be facilitated by an indicator, a substance that changes color at a specific pH level or concentration. Common indicators include phenolphthalein, which turns from colorless to pink, and methyl orange, which shifts from red to yellow.
The primary goal of a titration test is to determine the molarity of the unknown solution
. The relationship between the quantities can be described by the formula\[ M_1V_1 = M_2V_2 \]
Where \( M_1 \) and \( M_2 \) are the molarities of the unknown and known solutions, respectively, and \( V_1 \) and \( V_2 \) are their respective volumes. This equation assumes that the reaction proceeds to completion and that the stoichiometry of the reaction is well understood.
titration test
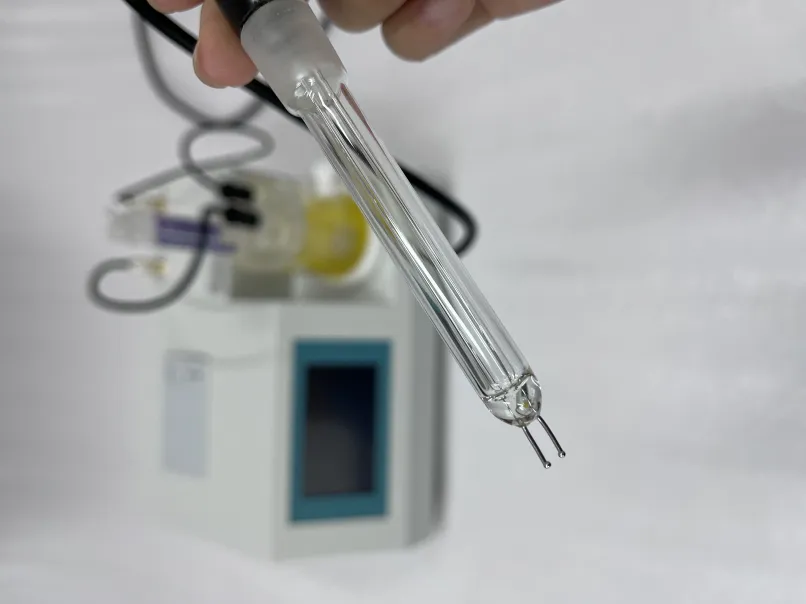
Titration can be classified into various types depending on the nature of the reactants involved. The most common types include acid-base titrations, redox titrations, complexometric titrations, and precipitation titrations. Acid-base titrations are arguably the most straightforward and widely practiced, involving the neutralization reaction between an acid and a base. In contrast, redox titrations involve the transfer of electrons between two species, which can be more complex due to the involvement of oxidation states.
Conducting a titration test requires a systematic approach. First, proper preparation of the reagents is essential. The titrant must be standardized, meaning its concentration is accurately determined, usually by titrating it against a primary standard. The analyte is then measured into a flask, and a few drops of the appropriate indicator are added. The titrant is added gradually while continuously swirling the flask until the endpoint is reached, marked by a sustained color change.
One of the critical aspects of performing titration tests is the importance of precision and accuracy. The use of pipettes, burettes, and other calibrated equipment minimizes errors in measurement. It is also essential to perform repeat titrations to obtain a mean value and assess the variability of results. By calculating the average and standard deviation, chemists can evaluate the reliability and accuracy of their titration tests.
Titration tests not only reinforce fundamental concepts in chemistry but also promote skills such as critical thinking, problem-solving, and attention to detail. These are invaluable in scientific inquiry and experimentation. Furthermore, understanding titration has practical implications; for instance, titration methods are used in quality control processes in industries to ensure that products meet safety and regulatory standards.
In conclusion, titration tests form an integral part of quantitative analysis in chemistry. They demonstrate the interplay between theory and practical skills, providing insights into chemical behavior and reactions. As students and professionals alike hone their titration skills, they not only learn to measure concentrations accurately but also cultivate a deeper appreciation for the scientific method and its applications across various sectors. Whether in an educational setting or practical applications, mastering titration techniques is an essential tool for any aspiring chemist or scientist.
-
Ensuring SF₆ Gas Safety: Introducing PUSH’s Integrated SF₆ Analyzer for Dew Point, Purity, and Decomposition MonitoringNewsJul.10,2025
-
Exploring the Main Types of Industrial Endoscopes and Their Applications Across IndustriesNewsJul.04,2025
-
Testing Equipment Industry Sees Major Advancements in 2025: Smart & Precision Technologies Lead the WayNewsJun.06,2025
-
Applications of Direct Current Generators in Renewable Energy SystemsNewsJun.05,2025
-
Hipot Tester Calibration and Accuracy GuidelinesNewsJun.05,2025
-
Digital Circuit Breaker Analyzer Features and BenefitsNewsJun.05,2025



